Quality Assurance
MC Diagnostics is ISO 13485 Certified
MCD recognises the importance of a stringent quality management system to ensure the highest standard of products are delivered to the market. We are ISO 13485 certified, which integrates strict design control from the beginning of assay development, through transfer to production, product validation and finally launch.
Our staff are also experienced in FDA 510(k) premarket notification applications and we maintain all documentation to enable our collaborators to obtain CE marking or FDA clearance.
A copy of our ISO 13845 Certificate is available to download here:

Slide title
Write your caption hereButton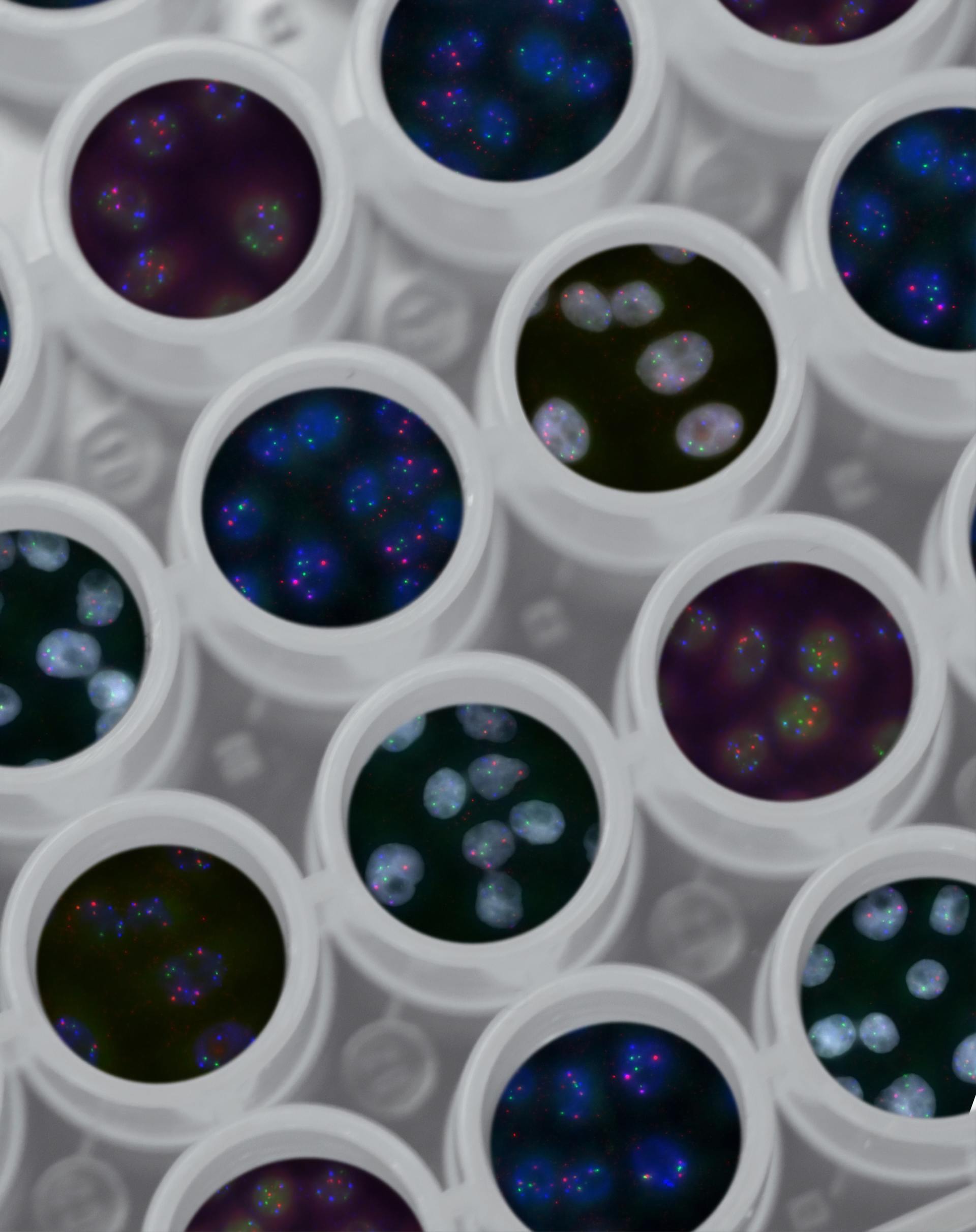
Slide title
Write your caption hereButton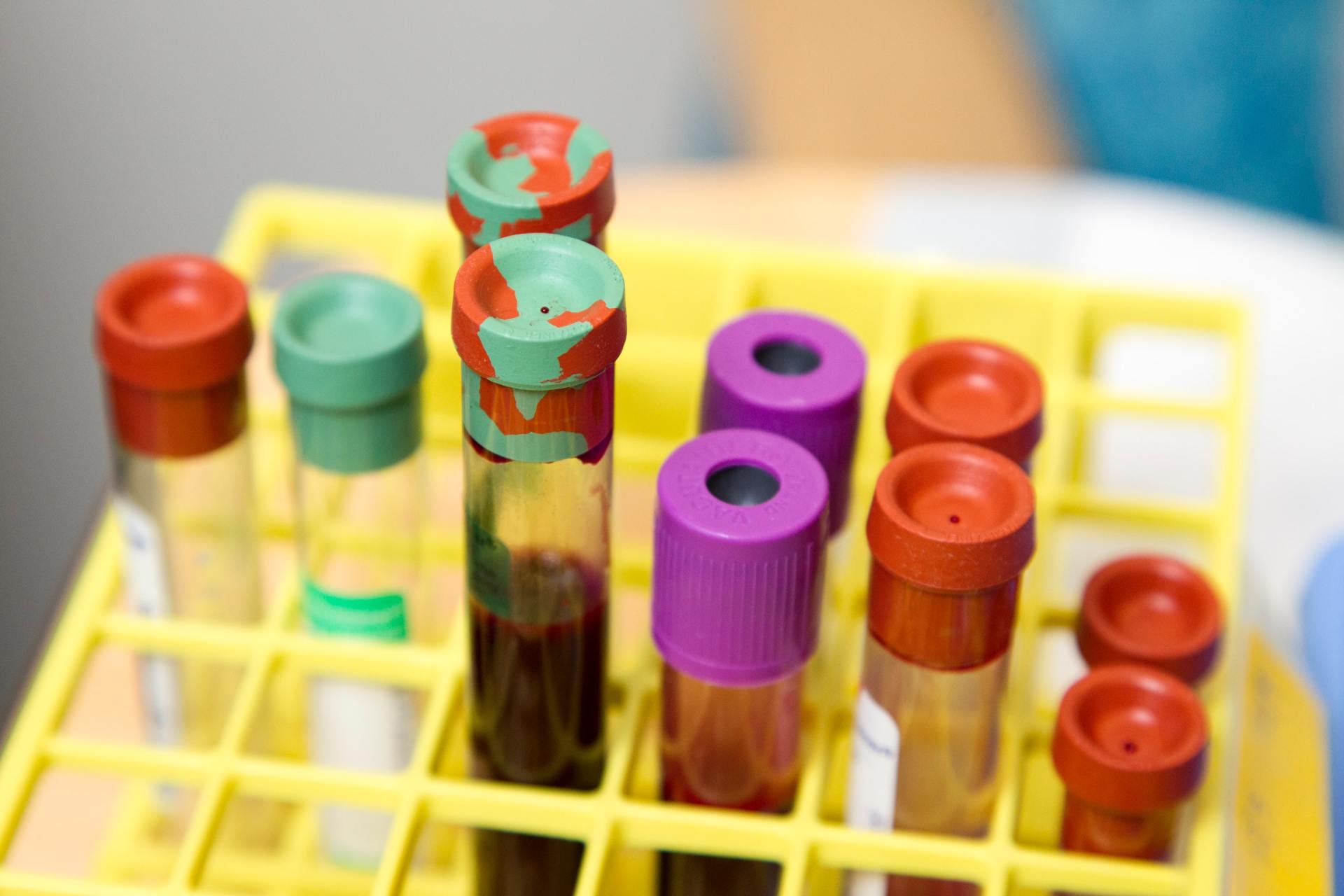
Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
Secure Online Payments
Satisfaction Guarantee
International Service
Premium Customer Service
Inclusive Kits
Customer Chat
Copyright © 2024 MC Diagnostics Ltd.
All Rights Reserved.
Registered in England & Wales
Company Number: 5843597.
Tel: +44 (0)151 2039898
MC Diagnostics Ltd
1 Hawkshead Road
Croft Business Park
Bromborough
Wirral
CH62 3RJ
United Kingdom





